ENGINEERING PROJECT HIGHLIGHTS
(Select Image to Expand)

with Data Acquisition

Workstation with Data Acquisition

Molding Machine

with Data Acquisition


For Tissue Heart Valves


Slit One Wall and Cut to Length

Vein Fixation

Functionality Tester

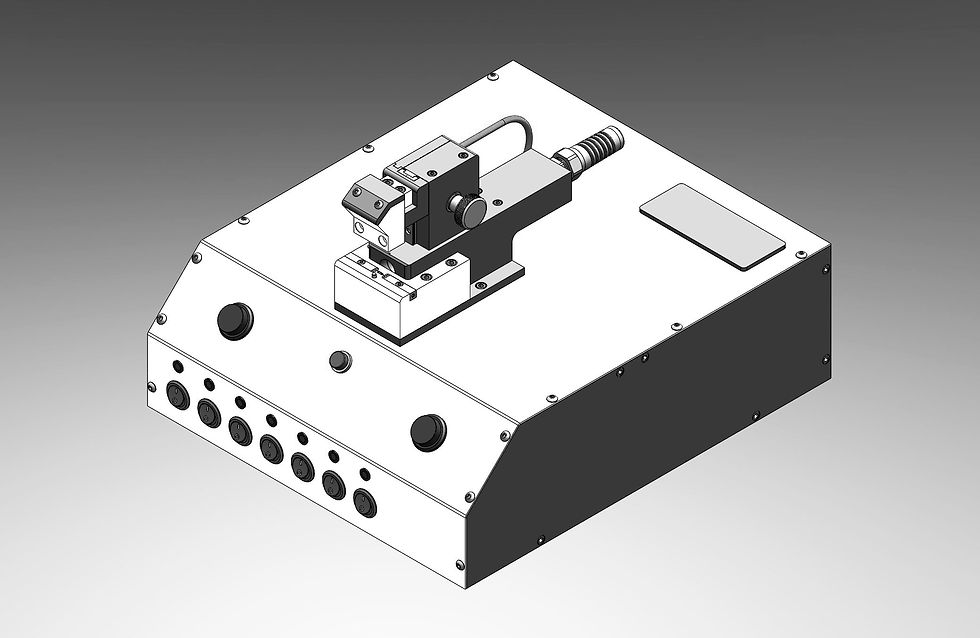
Test System with Data Acquisition

Tester

Analyzer

Workstation

Former


Pulse Duplicator

Stent Cloth Former

Assembly







Folding Fixture



Throughout my career, I've had the pleasure of working alongside a diverse group of medical device manufacturers ranging from early-stage, venture-backed startups to globally recognized Fortune 500 corporations. My career has centered on delivering high-value electro-mechanical design expertise and advanced process automation solutions that support both R&D initiatives and full-scale production environments. I bring cross-disciplinary expertise into the unique technical, regulatory, and operational demands of Class I, II, and III medical devices, ensuring that every solution I develop strengthens product quality, manufacturability, and reliability.
I specialize in designing and implementing innovative manufacturing and test systems that enable companies to scale efficiently while maintaining strict engineering and quality standards. From early concept development through full project implementation, I manage the complete lifecycle of custom equipment, fixtures, automated systems, and integrated test solutions. My hands-on engineering approach is grounded in strong attention to technical detail, cross-functional collaboration, and an ongoing commitment to continuous improvement.
Over the years, I’ve built a reputation as a technical leader capable of diagnosing complex manufacturing challenges, uncovering root causes, and delivering cost-effective solutions that improve yields, increase throughput, and reduce operational risk. Whether developing automated assembly and test systems, designing custom control interfaces, or supporting validation activities, my goal is always the same: to provide reliable, scalable, and compliant engineering solutions that accelerate product success.
AREAS OF EXPERTISE
Technical Leadership
Automated Test & Assembly Systems
Custom Laboratory & R&D Equipment
Manual and Automated Fixture Design
Integration of A.I. Tools into Process Engineering Workflows
Reliability Engineering & Yield Improvement
Cost Reduction Strategies
Vendor Qualification
Process Validation Support (IQ, OQ, PQ)
Technical Documentation & Engineering Reports
Control System & Electrical Schematic Design
Data Acquisition Systems
PLC Programming (Ladder Logic)
Custom Software Interface (LabVIEW)
Machining & Precision Fabrication
3D Printing: SLA & Fused Filament Fabrication
Prototype Mold Design
SolidWorks & AutoCAD
Advanced Technical Troubleshooting

PROFESSIONAL PROFILE
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•














JenaValve Technology, Inc. - Irvine, CA.
Transcatheter Aortic Valve Replacement (TAVR) for severe aortic
regurgitation and stenosis
Johnson & Johnson, Corp., Biosense Webster - Irvine, CA.
Diagnostic and treatment tools for cardiac arrhythmia
Edwards Lifesciences, Corp. - Irvine, CA.
Surgical devices for the treatment of advanced cardiovascular disease
Medtronic, Inc. - Santa Ana, CA.
Cardiac valve prosthesis, tissue
Smith & Nephew PLC., Sports Medicine Division - Irvine, CA.
Suturing device for arthroscopic rotator cuff repair surgery
Merit Medical Systems, Inc., Cianna Medical - Aliso Viejo, CA.
Technologies for early-stage breast cancer treatment
Radiance Medical Systems, Inc. - Irvine, CA.
Intravascular radiation therapy catheter
3M, Inc., CDI Division - Tustin, CA.
Blood gas analyzer
Boston Scientific, Corp., Guidant, Corp. - Menlo Park, CA.
Surgical devices for cardio-thoracic and OBGYN surgery, catheters, trocar
Becton Dickinson and Co., Luther Medical Products, Inc. - Tustin, CA.
Intravascular catheters, split needle cannula, devices for vascular access
and infusion therapy
Arbor Surgical Technologies, Inc. - Irvine, CA.
Cardiac valve prosthesis, tissue
Endologix, Inc. - Irvine, CA.
Aortic aneurysm stent graph catheter delivery system
Applied Medical, Inc. - Rancho Santa Margarita, CA.
Surgical products for general surgery, urology, vascular, cardiac, colorectal
and OBGYN surgery
Sub-Q Medical, Inc. - San Clemente, CA.
Subcutaneous wound management device
EV3 Neurovascular, Inc., Micro Therapeutics, Inc. - Irvine, CA.
Infusion catheters, guidewire
USGI Medical - San Clemente, CA.
Endoscopic access device, suture anchors
CR Bard, Inc., Venetech Intl., Inc. - San Diego, CA.
Intravascular catheter securement system, IV fluid transfer unit
Micra-Pro, Inc. - Paradise, CA.
Micro abrasive dental polishing disk
Geiger Medical Technologies - Monarch Beach, CA.
Handheld cauterizing device
CLIENT COMPANY PROFILES
PROJECT WORKFLOW
My approach to medical device process development is grounded in engineering partnership rather than product sales. I provide specialized engineering and design services, billed on an hourly basis, to help organizations develop, refine, and validate their manufacturing and testing processes. Equipment and fixtures are custom designed for specific client projects and are not marketed or sold as standalone products. This model allows clients to retain full ownership and control of all project hardware and expenses, with items such as machining, metal processing, components, and assembly or troubleshooting activities procured directly through the client’s facility or approved suppliers. Project workflows are intentionally flexible and are tailored to the scope, technical risk, and regulatory complexity of each engagement, ensuring that the development process aligns with both practical manufacturing needs and quality system requirements.

Password
Forgot Password?
Don't have an account?
Sign Up
WELCOME TO YOUR
PROJECT PORTAL
This secure space provides centralized access to project schedules, technical documentation, project status updates,
video call scheduling, and payment processing.

